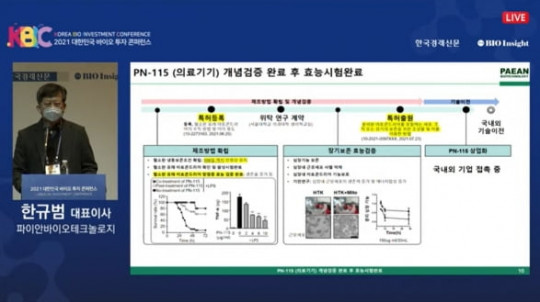
Pian Biotechnology has officially launched the world’s first clinical trial of a mitochondrial therapy, a major advancement in regenerative medicine. The therapy, PN-101, is engineered to restore mitochondrial function, improve cellular energy production, and potentially treat degenerative diseases and conditions associated with mitochondrial dysfunction.
Following promising preclinical results, PN-101 received regulatory approval to enter Phase 1 clinical trials. This study will focus on evaluating safety, tolerability, and early efficacy in a small patient cohort. Scientists believe that targeting mitochondrial dysfunction can address underlying causes of aging-related and chronic diseases, making PN-101 a potentially transformative therapy.
The CEO of Pian Biotechnology highlighted the historic nature of the trial: “Launching the first clinical trial for a mitochondrial therapy is a pivotal moment in medicine. We aim to deliver innovative treatments that can improve patient quality of life worldwide.”
The company plans to expand into later-phase trials to optimize dosing and explore broader therapeutic applications. PN-101 represents a first-in-class mitochondrial therapy, opening new frontiers in regenerative medicine. This clinical trial not only sets a global precedent but also demonstrates the growing potential of mitochondria-targeted therapies to revolutionize treatment for degenerative diseases.