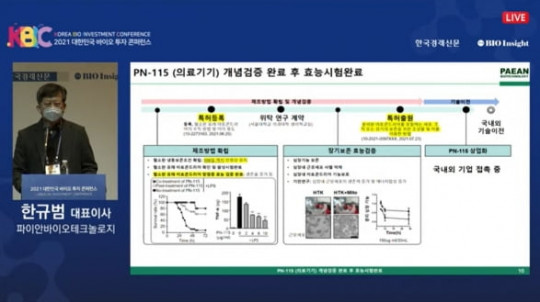
Pian Biotechnology has officially launched the world’s first clinical trial of a mitochondrial therapy, marking a major advancement in regenerative medicine. The therapy, PN-101, targets mitochondrial dysfunction—a key factor in degenerative and chronic diseases—by restoring cellular energy production and improving overall cell function.
Following successful preclinical studies, PN-101 has received approval for Phase 1 clinical trials, which will assess safety, tolerability, and early efficacy in a controlled group of patients. The trial aims to measure mitochondrial activity, cellular energy levels, and markers of degeneration, providing valuable data for future clinical development.
The CEO of Pian Biotechnology stated, “Launching the first mitochondrial therapy trial is a pivotal moment in medicine. We aim to deliver innovative treatments that can improve patients’ quality of life worldwide.”
With plans for future Phase 2 and 3 trials, PN-101 could become a first-in-class therapy, offering a new approach to treat conditions that currently have limited options. This clinical trial not only sets a global precedent but also demonstrates the growing promise of mitochondria-targeted therapies in regenerative medicine, opening new horizons for treating degenerative diseases and aging-related disorders.