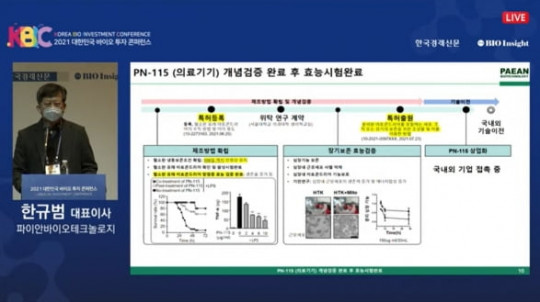
Pian Biotechnology’s PN-101 is set to redefine regenerative medicine as the first mitochondrial therapy entering clinical trials. Targeting the cellular powerhouses, PN-101 aims to restore energy production, improve cell function, and combat degenerative diseases.
Mitochondrial dysfunction is linked to aging, neurodegenerative disorders, and chronic metabolic conditions. By addressing the root cause, PN-101 offers a novel therapeutic approach that could surpass conventional treatments focused only on symptoms.
The ongoing Phase 1 clinical trial will evaluate safety, tolerability, and early efficacy, providing critical insights for future development. Researchers are optimistic that PN-101 can open new possibilities for preventing and treating degenerative conditions at the cellular level.
Looking ahead, Pian Biotechnology plans to expand trials globally and explore combination therapies with other regenerative strategies. PN-101 has the potential to become a first-in-class therapy, inspiring further innovation in mitochondrial medicine and establishing a new standard for regenerative treatments worldwide.