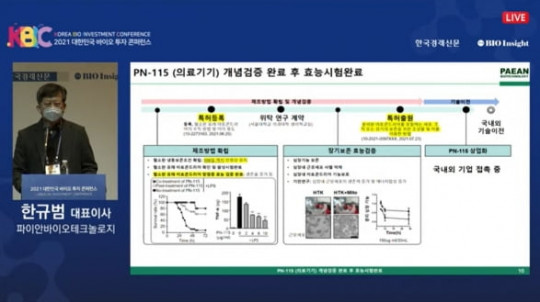
Pian Biotechnology’s PN-101 has become the first mitochondrial therapy to enter clinical trials, establishing a global precedent in regenerative medicine. The therapy targets mitochondrial dysfunction, which is a major factor in aging, neurodegeneration, and metabolic diseases.
The Phase 1 trial will evaluate safety and preliminary efficacy in a controlled patient cohort. By restoring mitochondrial function, PN-101 aims to improve cellular energy metabolism and potentially slow or reverse degenerative processes.
Experts in regenerative medicine have hailed the trial as a landmark development. “Mitochondria-targeted therapies have long been a scientific goal, and PN-101 represents the first step in translating this concept into clinical practice,” said a research consultant.
Pian Biotechnology plans to expand clinical studies to later phases, exploring broader applications and optimized dosing. PN-101’s progress highlights the emerging potential of mitochondrial medicine, paving the way for therapies that address the root cause of cellular degeneration rather than only treating symptoms.
This milestone reinforces Pian Biotechnology’s position as a pioneer in regenerative therapies, bringing new hope to patients worldwide.